우리 일상 생활에서, 따뜻한 햇살과 별개로, LED 조명이 주요 조명원이 되었습니다.. 늘 우리와 함께하는 휴대폰 화면부터 아늑하고 편안한 홈 조명까지, 그리고 밤에는 도시를 장식하는 눈부신 네온 불빛까지, 독특한 매력으로 사람들을 사로잡는 LED 조명. 그래서, 왜 LED 조명이 빛을 방출할 수 있습니까?, 그리고 어떻게 흰색처럼 풍부한 색상을 표현할 수 있을까요?, 녹색, 파란색, 그리고 빨간색? 이 현상 뒤에는 어떤 과학적 미스터리가 숨어 있나요?? 더 깊이 탐구하고 함께 탐구해 봅시다..
LED가 빛을 낼 수 있는 이유?
LED는 실제로는 발광다이오드이다., 반도체 재료로 만든 것. LED의 빛 방출 능력 뒤에 있는 원리는 고유한 에너지 밴드 구조와 캐리어 재결합 과정을 기반으로 합니다..
반도체 재료는 원자가 공간에 주기적으로 배열되어 있는 고품질의 결정체입니다.. 각 원자는 양전하를 띤 핵과 음전하를 띤 전자로 구성됩니다.. 결정에는 많은 수의 원자가 포함되어 있기 때문에, 이 원자들은 서로 상호작용한다. 이 주기적인 상호작용은 결정 내에 에너지 밴드를 형성합니다., 그리고 수많은 에너지 밴드가 존재합니다. 각 에너지 밴드는 전자가 차지할 수 있는 많은 위치를 제공합니다., 에너지 대역 내 전자의 분포는 가장 낮은 에너지 위치에서 더 높은 에너지 위치로 진행됩니다..

결정의 에너지 밴드 내에서, 일부는 전자로 채워져 있고 다른 일부는 전자로 채워져 있지 않습니다.. 점유된 에너지 밴드 중, 에너지가 가장 높은 사람, 전자가 부분적으로만 채워져 있는 경우, 전도대로 알려져 있다. 전도대의 전자는 상대적으로 높은 에너지 준위에 존재하며 자유롭게 움직일 수 있을 만큼 충분한 에너지를 가지고 있습니다., 그로 인해 전류가 형성된다. 거꾸로, 만약 이 에너지 밴드가 전자로 완전히 채워져 있다면, 이를 가전자대라고 한다. 가전자대에 있는 전자는 에너지 준위가 더 낮습니다., 자유롭게 움직일 수 있을 만큼 에너지가 부족합니다.. 가전자대 상단과 전도대 하단 사이에는 일정한 에너지 간격이 있습니다., 밴드갭 또는 금지된 밴드로 알려져 있음. 전자는 이 영역에 존재할 수 없습니다, 비록 그들이 그것을 횡단할 수는 있지만.
외부 전압을 인가하는 경우, 가전자대의 일부 전자는 전도대로 여기될 수 있음, 자유롭게 움직이는 전하 캐리어 형성. 그 동안에, 원래 완전히 점유된 원자가대에서, 일부 전자가 없으면 빈 위치가 남습니다., 우리가 구멍이라고 부르는 곳. 반도체 재료 내에서 이러한 전하 캐리어의 이동 및 재결합 과정은 빛 방출을 달성하는 데 중요합니다..
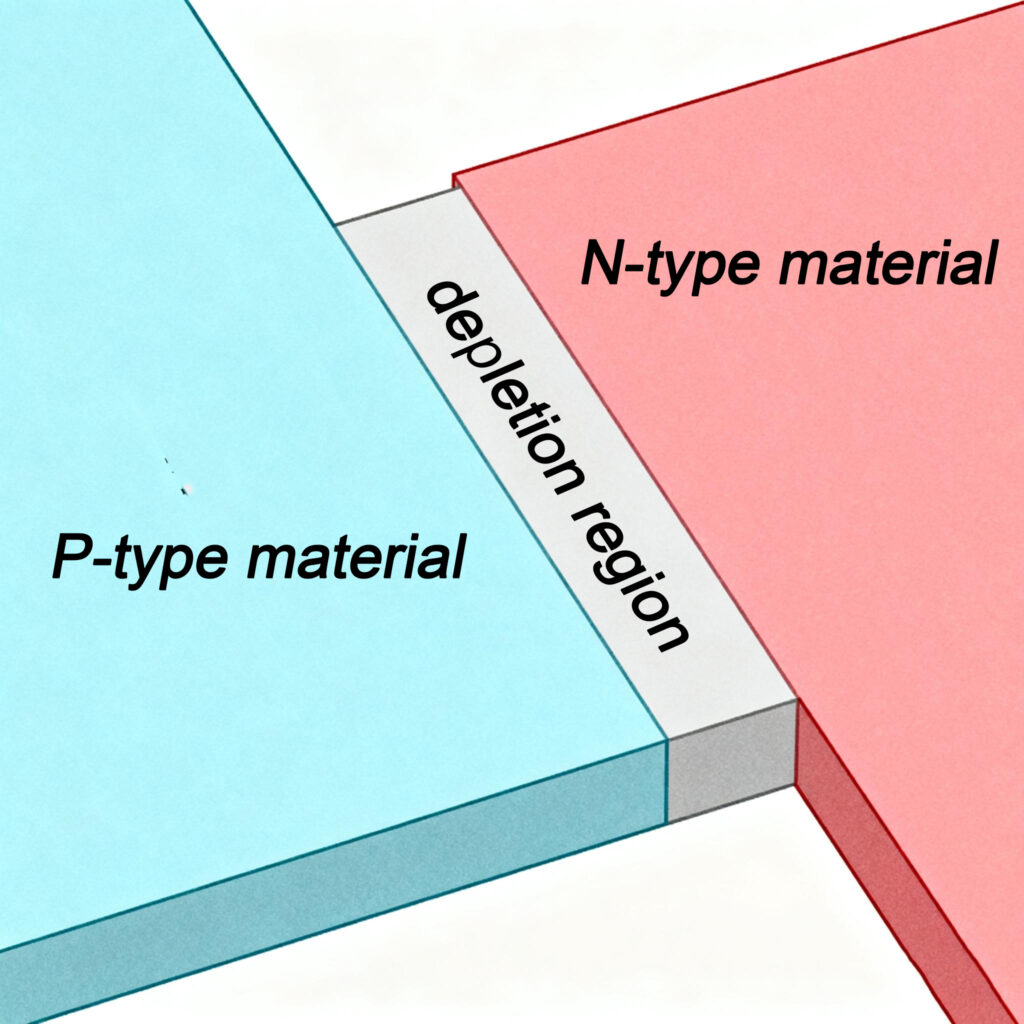
LED는 발광다이오드이다., P형 반도체와 N형 반도체를 결합하여 형성된 PN 접합을 갖는 다이오드 구조로 구성. P형 반도체와 N형 반도체를 함께 배치하면, 회로에 연결하지 않고도, 일부 전자는 N형 반도체에서 P형 반도체로 확산되어 P형 물질의 정공으로 떨어집니다.. 이로 인해 P형 반도체는 약간의 음전하를 띠게 됩니다., 반면 N형 반도체는 약간 양전하를 띠게 됩니다., 결과적으로 내부 전기장이 형성됩니다., PN 접합으로 알려져 있음. PN 접합 내, 전자가 구멍을 만날 때, 전도대에 있는 전자는 가전자대에 있는 정공으로 이동합니다.. 이 전자-정공 재결합 과정에서, 에너지는 빛의 형태로 방출된다.. 이것이 LED가 빛을 방출하는 기본 원리입니다..
LED 조명은 다양한 색상의 빛을 방출합니다.
발광 과정에 관여하는 에너지 준위는 반도체 소재의 에너지 밴드 구조와 관련이 있습니다.. 재료와 에너지 밴드 구조가 다르면 방출 파장과 색상이 달라집니다.. 구체적으로:
전자 전이와 에너지 차이
전자가 에너지가 높은 오비탈에서 에너지가 낮은 오비탈로 전이할 때, 전자기파의 형태로 전파되는 에너지를 방출합니다.. 전자 전이 중에 방출되는 에너지 차이는 요소마다 다릅니다., 다양한 파장의 빛에 대응. 에너지가 클수록, 파장이 짧을수록. 스펙트럼에서, 필요한 에너지는 왼쪽에서 오른쪽으로 증가합니다.. 이것이 빨간색 LED가 최초로 발명된 이유입니다., 최소한의 에너지만 필요하기 때문에, 그다음에는 녹색, 그리고 파란색. 이것이 바로 청색 LED의 발명이 도전적인 이유이기도 합니다., 블루라이트는 더 많은 에너지를 필요로 하기 때문에.
파장 400-500 나노미터는 청색광에 해당합니다., 500-600 나노미터에서 녹색광으로, 그리고 600-700 나노미터를 적색광으로.
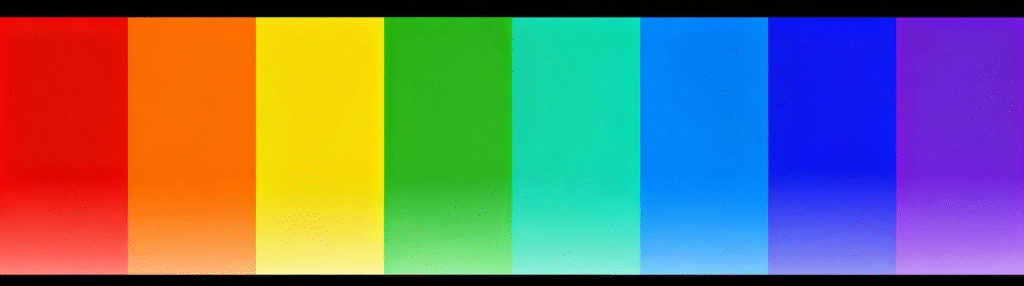
재료 선택 및 색상 제어
LED 내부의 PN 접합은 일반적으로 갈륨 비소 인화물과 같은 화합물로 구성됩니다.. 다양한 재료를 사용하는 경우, 대전 시 방출되는 빛의 파장은 다양합니다.. 그러므로, 내부 PN 접합의 재질을 간단히 변경함으로써, 다양한 색상의 발광다이오드 제작 가능.
파란색 LED: 질화갈륨을 반도체 재료로 사용하는 경우, 전자 전이 에너지가 상대적으로 높다, 대략 파장의 빛을 방출합니다. 460 나노미터, 파란색으로 보이는 것. 이것이 파란색 LED가 방출되는 원리입니다..
녹색 LED: 인화갈륨을 사용하는 경우, 방출된 빛은 약 560 나노미터, 그린 컬러를 선보이며. 녹색 LED의 방출 메커니즘을 설명합니다..
빨간색 LED: 갈륨비소, 반면에, 대략 파장의 빛을 방출합니다. 660 나노미터, 빨갛게 보이는. 이것이 빨간색 LED가 발광하는 원리입니다..
LED를 사용하여 백색광을 생성하려면, 백색 LED라고도 함, 추가 디자인이 필요합니다. 한 가지 방법은 서로 다른 색상의 발광 다이오드를 결합하여 백색광 효과를 만드는 것입니다.. 또 다른 접근 방식은 형광체로 코팅된 파란색 LED를 활용합니다.. LED에서 방출되는 청색광이 형광체를 조명합니다., 청색광의 일부를 다른 색상의 빛으로 변환. 최종 효과는 백색광으로 나타납니다..
LED의 발광 원리와 다양한 색상의 발광 메커니즘에 대한 위의 자세한 소개를 통해, 이제 모두가 LED에 대해 더 깊이 이해하게 되었다고 생각합니다.. 지속적인 기술 발전으로, 조명, 디스플레이 등 다양한 분야에서 LED의 활용이 더욱 확대되고 눈부시게 될 것입니다..





