In unserem täglichen Leben, Abgesehen vom warmen Sonnenlicht, LED-Leuchten sind zur Hauptbeleuchtungsquelle geworden. Von den Handybildschirmen, die uns jederzeit begleiten, bis hin zur gemütlichen und komfortablen Heimbeleuchtung, und dann zu den schillernden Neonlichtern, die nachts Städte schmücken, LED-Leuchten bestechen durch ihren einzigartigen Charme. Also, Warum genau können LED-Leuchten Licht ausstrahlen?, und wie können sie eine so reiche Farbpalette wie Weiß darstellen?, Grün, Blau, und rot? Welche wissenschaftlichen Geheimnisse stecken hinter diesem Phänomen?? Lassen Sie uns tiefer eintauchen und gemeinsam erkunden.
Warum können LEDs Licht ausstrahlen??
Eine LED ist eigentlich eine Leuchtdiode, welches aus Halbleitermaterialien besteht. Das Prinzip hinter der Fähigkeit einer LED, Licht zu emittieren, basiert auf ihrer einzigartigen Energiebandstruktur und dem Ladungsträgerrekombinationsprozess.
Halbleitermaterialien sind hochwertige Kristalle, in denen Atome periodisch im Raum angeordnet sind. Jedes Atom besteht aus einem positiv geladenen Kern und negativ geladenen Elektronen. Da ein Kristall eine große Anzahl von Atomen enthält, Diese Atome interagieren miteinander. Diese periodische Wechselwirkung bildet Energiebänder innerhalb des Kristalls, und es sind zahlreiche Energiebänder vorhanden. Jedes Energieband bietet den Elektronen viele Positionen, die sie einnehmen können, und die Verteilung der Elektronen innerhalb eines Energiebandes schreitet von den Positionen mit der niedrigsten Energie zu denen mit höherer Energie voran.

Innerhalb der Energiebänder eines Kristalls, Einige sind mit Elektronen besetzt, andere nicht. Unter den besetzten Energiebändern, derjenige mit der höchsten Energie, wenn es nur teilweise mit Elektronen gefüllt ist, wird als Leitungsband bezeichnet. Elektronen im Leitungsband befinden sich auf einem relativ hohen Energieniveau und verfügen über ausreichend Energie, um sich frei zu bewegen, Dadurch entsteht ein elektrischer Strom. Umgekehrt, wenn dieses Energieband vollständig mit Elektronen gefüllt ist, es wird Valenzband genannt. Elektronen im Valenzband befinden sich auf niedrigeren Energieniveaus, und ihre Energie reicht nicht aus, um sich frei bewegen zu können. Zwischen dem oberen Ende des Valenzbandes und dem unteren Ende des Leitungsbandes besteht eine gewisse Energielücke, bekannt als Bandlücke oder verbotene Band. Elektronen können sich in dieser Region nicht aufhalten, obwohl sie es durchqueren können.
Wenn eine externe Spannung angelegt wird, Einige Elektronen im Valenzband können in das Leitungsband angeregt werden, Bildung frei beweglicher Ladungsträger. In der Zwischenzeit, im ursprünglich voll besetzten Valenzband, Das Fehlen einiger Elektronen hinterlässt freie Positionen, die wir als Löcher bezeichnen. Die Bewegungs- und Rekombinationsprozesse dieser Ladungsträger innerhalb des Halbleitermaterials sind entscheidend für die Erzielung der Lichtemission.
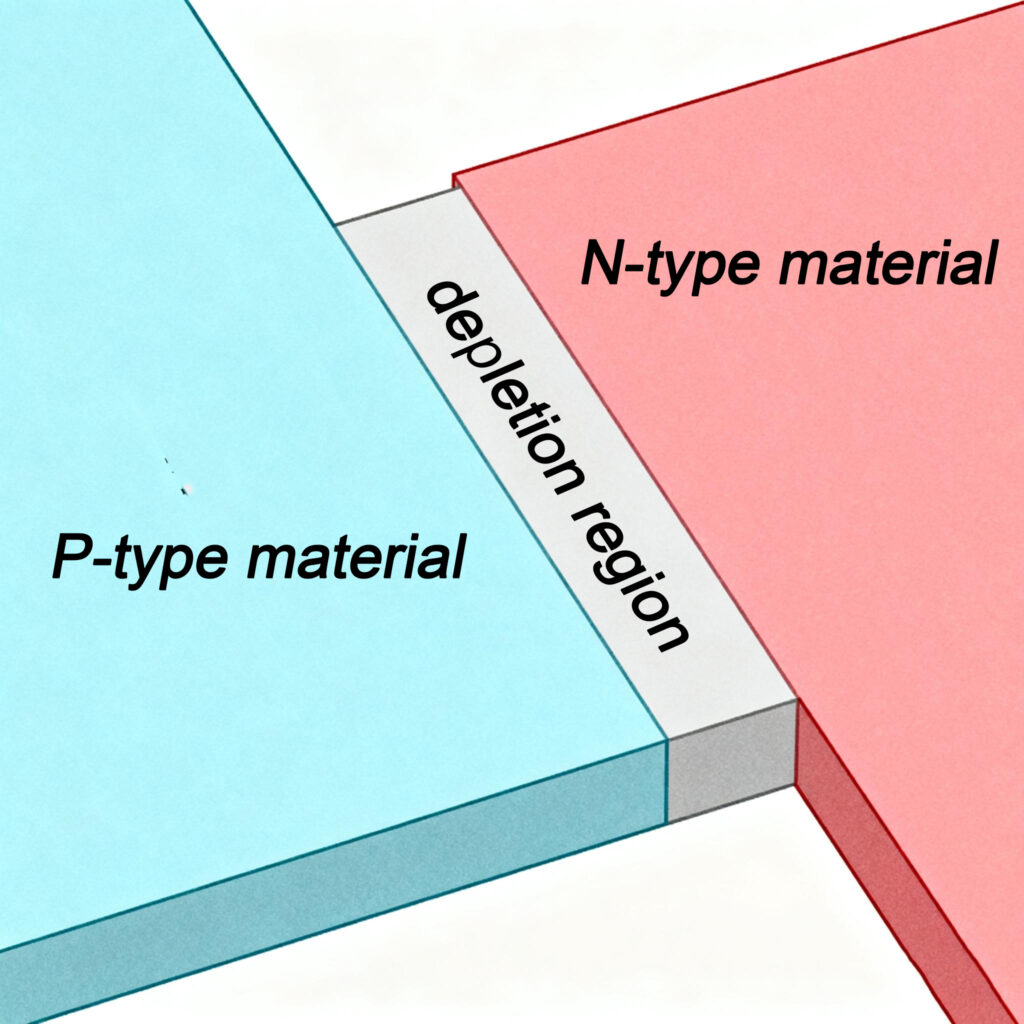
Eine LED ist eine Leuchtdiode, Sie besteht aus einer Diodenstruktur mit einem PN-Übergang, der durch die Kombination von P-Typ- und N-Typ-Halbleitern gebildet wird. Wenn wir die Halbleiter vom P-Typ und N-Typ zusammen platzieren, auch ohne sie an einen Stromkreis anzuschließen, Einige Elektronen diffundieren vom N-Typ-Halbleiter zum P-Typ-Halbleiter und fallen in die Löcher im P-Typ-Material. Dadurch erhält der Halbleiter vom P-Typ eine leichte negative Ladung, während der Halbleiter vom N-Typ leicht positiv geladen wird, Dadurch entsteht ein inneres elektrisches Feld, bekannt als PN-Übergang. Innerhalb der PN-Kreuzung, wenn Elektronen auf Löcher treffen, Elektronen im Leitungsband springen zu den Löchern im Valenzband. Während dieses Elektron-Loch-Rekombinationsprozesses, Energie wird in Form von Licht freigesetzt. Dies ist das Grundprinzip der Lichtemission von LEDs.
LED-Leuchten geben Licht in verschiedenen Farben ab
Das am Lichtemissionsprozess beteiligte Energieniveau hängt von der Energiebandstruktur des Halbleitermaterials ab. Unterschiedliche Materialien und Energiebandstrukturen führen zu unterschiedlichen Emissionswellenlängen und -farben. Speziell:
Elektronenübergang und Energiedifferenz
Wenn ein Elektron von einem Orbital höherer Energie in ein Orbital niedrigerer Energie übergeht, Es setzt Energie frei, die sich in Form elektromagnetischer Wellen ausbreitet. Die bei Elektronenübergängen freigesetzte Energiedifferenz variiert zwischen den verschiedenen Elementen, entsprechend Licht unterschiedlicher Wellenlänge. Je größer die Energie, je kürzer die Wellenlänge. Im Spektrum, der Energiebedarf nimmt von links nach rechts zu. Aus diesem Grund wurden zuerst rote LEDs erfunden, da sie am wenigsten Energie benötigen, gefolgt von Grün, und dann blau. Aus diesem Grund war die Erfindung der blauen LEDs auch eine Herausforderung, da blaues Licht mehr Energie benötigt.
Wellenlängen von 400-500 Nanometer entsprechen blauem Licht, 500-600 Nanometer in grünes Licht, Und 600-700 Nanometer in rotes Licht.
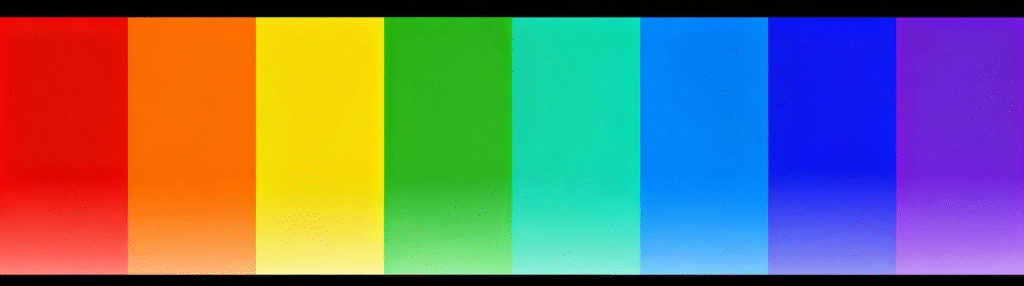
Materialauswahl und Farbkontrolle
Der PN-Übergang innerhalb einer LED besteht im Allgemeinen aus Verbindungen wie Galliumarsenidphosphid. Wenn unterschiedliche Materialien verwendet werden, die Wellenlängen des emittierten Lichts bei der Elektrifizierung variieren. daher, durch einfaches Ändern des Materials des internen PN-Übergangs, Es können Leuchtdioden unterschiedlicher Farbe hergestellt werden.
Blaue LED: Als Halbleitermaterial wird Galliumnitrid verwendet, die Elektronenübergangsenergie ist relativ hoch, Dabei wird Licht mit einer Wellenlänge von ca 460 Nanometer, welches blau erscheint. Dies ist das Prinzip hinter der Emission blauer LEDs.
Grüne LED: Wenn Galliumphosphid verwendet wird, Das emittierte Licht hat eine Wellenlänge von ca 560 Nanometer, präsentiert eine grüne Farbe. Dies erklärt den Emissionsmechanismus grüner LEDs.
Rote LED: Galliumarsenid, auf der anderen Seite, gibt Licht mit einer Wellenlänge von ca 660 Nanometer, rot erscheinen. Dies ist das Prinzip, das der Emission roter LEDs zugrunde liegt.
Zur Erzeugung von weißem Licht mit LEDs, auch als weiße LEDs bekannt, Es sind zusätzliche Designs erforderlich. One method involves combining light-emitting diodes of different colors to create a white light effect. Another approach utilizes a blue LED coated with phosphor. The blue light emitted by the LED illuminates the phosphor, converting a portion of the blue light into other colors of light. The final effect appears as white light.
Through the detailed introduction above on the light-emitting principle of LEDs and the mechanisms behind their emission of different colors, I believe everyone now has a deeper understanding of LEDs. With continuous technological advancements, the applications of LEDs in various fields such as lighting and displays will become even more widespread and remarkable.





