في حياتنا اليومية, بصرف النظر عن أشعة الشمس الدافئة, أصبحت مصابيح LED المصدر الرئيسي للإضاءة. From the mobile phone screens that accompany us at all times to the cozy and comfortable home lighting, and then to the dazzling neon lights that adorn cities at night, LED lights captivate people with their unique charm. لذا, why exactly can LED lights emit light, and how are they able to display such a rich array of colors like white, green, أزرق, and red? What scientific mysteries lie behind this phenomenon? Let’s delve deeper and explore together.
Why can LEDs emit light?
An LED is actually a light-emitting diode, which is made of semiconductor materials. The principle behind an LED’s ability to emit light is based on its unique energy band structure and the carrier recombination process.
Semiconductor materials are high-quality crystals in which atoms are arranged periodically in space. Each atom consists of a positively charged nucleus and negatively charged electrons. Since a crystal contains a large number of atoms, these atoms interact with one another. This periodic interaction forms energy bands within the crystal, and there are numerous energy bands present. Each energy band provides many positions for electrons to occupy, and the distribution of electrons within an energy band progresses from the lowest energy positions to those with higher energy.

Within the energy bands of a crystal, some are occupied by electrons while others are not. Among the occupied energy bands, the one with the highest energy, if only partially filled with electrons, is known as the conduction band. Electrons in the conduction band reside at relatively high energy levels and possess sufficient energy to move freely, thereby forming an electric current. على العكس من ذلك, if this energy band is completely filled with electrons, it is called the valence band. Electrons in the valence band are at lower energy levels, and their energy is insufficient to allow them to move freely. There is a certain energy gap between the top of the valence band and the bottom of the conduction band, known as the bandgap or forbidden band. Electrons cannot reside in this region, although they can traverse it.
When an external voltage is applied, some electrons in the valence band can be excited to the conduction band, forming freely moving charge carriers. في أثناء, in the originally fully occupied valence band, the absence of some electrons leaves vacant positions, which we refer to as holes. The movement and recombination processes of these charge carriers within the semiconductor material are crucial for achieving light emission.
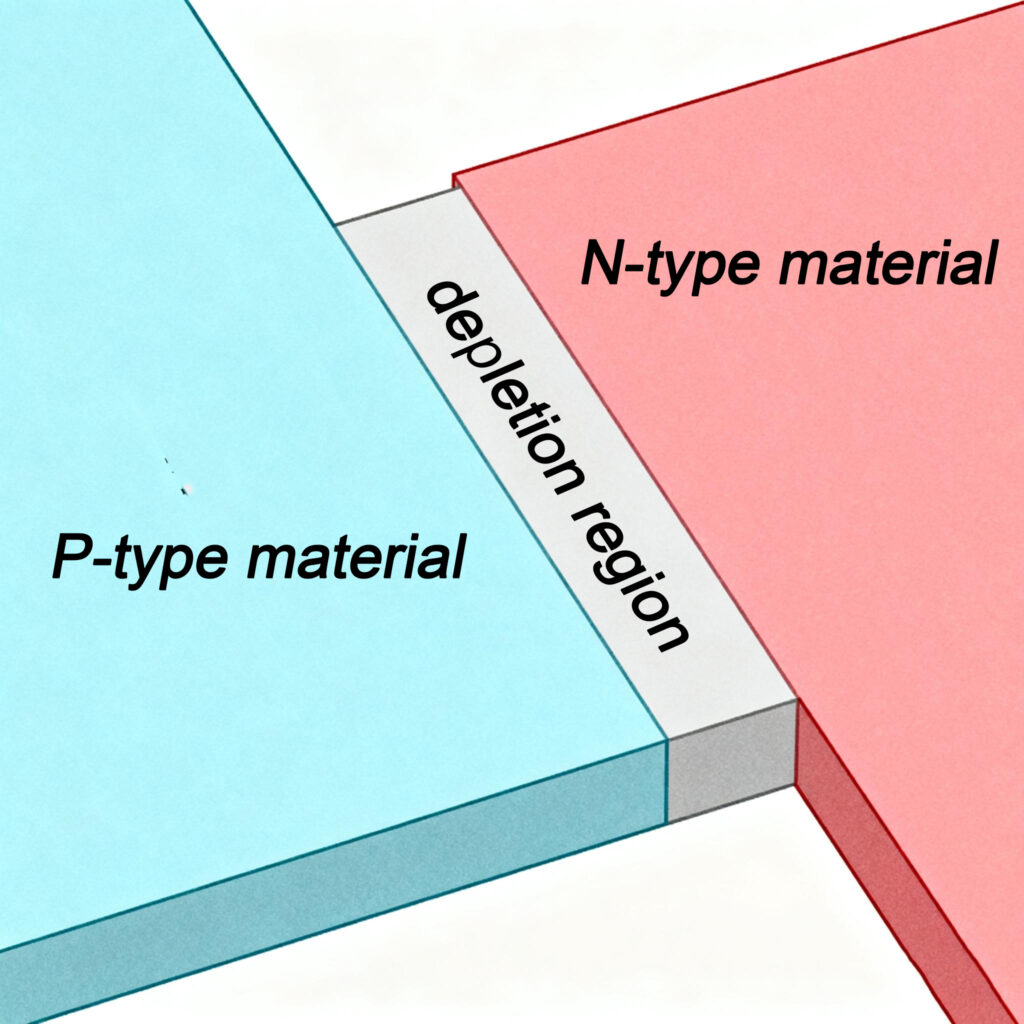
An LED is a light-emitting diode, which consists of a diode structure with a PN junction formed by combining P-type and N-type semiconductors. When we place the P-type and N-type semiconductors together, even without connecting them to a circuit, some electrons will diffuse from the N-type semiconductor to the P-type semiconductor and fall into the holes in the P-type material. This causes the P-type semiconductor to acquire a slight negative charge, while the N-type semiconductor becomes slightly positively charged, resulting in the formation of an internal electric field, known as the PN junction. Within the PN junction, when electrons encounter holes, electrons in the conduction band will jump to the holes in the valence band. During this electron-hole recombination process, energy is released in the form of light. This is the fundamental principle behind how LEDs emit light.
LED lights emit light of different colors
The energy level involved in the light-emitting process is related to the energy band structure of the semiconductor material. Different materials and energy band structures result in different emission wavelengths and colors. Specifically:
Electron Transition and Energy Difference
When an electron transitions from a higher-energy orbital to a lower-energy orbital, it releases energy that propagates in the form of electromagnetic waves. The energy difference released during electron transitions varies among different elements, corresponding to light of different wavelengths. The greater the energy, the shorter the wavelength. In the spectrum, the energy required increases from left to right. This is why red LEDs were invented first, as they require the least amount of energy, followed by green, and then blue. This is also why the invention of blue LEDs was challenging, as blue light requires more energy.
Wavelengths of 400-500 nanometers correspond to blue light, 500-600 nanometers to green light, و 600-700 nanometers to red light.
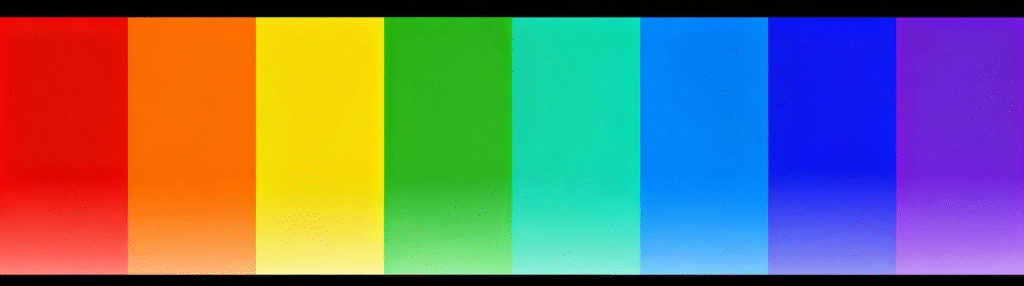
Material Selection and Color Control
The PN junction inside an LED is generally composed of compounds such as gallium arsenide phosphide. When different materials are used, the wavelengths of the emitted light upon electrification vary. لذلك, by simply changing the material of the internal PN junction, light-emitting diodes of different colors can be produced.
Blue LED: When gallium nitride is used as the semiconductor material, the electron transition energy is relatively high, releasing light with a wavelength of approximately 460 nanometers, which appears blue. This is the principle behind the emission of blue LEDs.
Green LED: When gallium phosphide is utilized, the emitted light has a wavelength of about 560 nanometers, presenting a green color. This explains the emission mechanism of green LEDs.
Red LED: Gallium arsenide, على الجانب الآخر, releases light with a wavelength of approximately 660 nanometers, appearing red. This is the principle underlying the emission of red LEDs.
To produce white light with LEDs, also known as white LEDs, additional designs are required. One method involves combining light-emitting diodes of different colors to create a white light effect. Another approach utilizes a blue LED coated with phosphor. The blue light emitted by the LED illuminates the phosphor, converting a portion of the blue light into other colors of light. The final effect appears as white light.
Through the detailed introduction above on the light-emitting principle of LEDs and the mechanisms behind their emission of different colors, I believe everyone now has a deeper understanding of LEDs. With continuous technological advancements, the applications of LEDs in various fields such as lighting and displays will become even more widespread and remarkable.